Optimizing Tuberculosis Treatment through Mathematical Modeling
Applying advanced mathematical models to enhance antibiotic effectiveness and reduce drug resistance.
Learn More About Our Research
The Struggles of Current Tuberculosis Treatment
Tuberculosis (TB) remains the bacterial infection with the highest disease burden worldwide. Approximately one third of the global population is latently infected, and although anti-tuberculosis drugs have been available for decades, the success rates of treatment remain poor. In the EU, the overall treatment success rate is only 74%, with limited improvements even in high-income countries like Norway or Switzerland, where success plateaus at around 85%. Furthermore, the appearance and spread of drug-resistant TB strains, such as MDR (multi-drug-resistant) and XDR (extensively drug-resistant) TB, has complicated control efforts that rely on standardized regimens.
When TB develops resistance to first-line drugs (MDR) or second-line drugs (XDR), the treatment success rates plummet to 40% to 34%, respectively. This stark contrast emphasizes the urgent need for more effective treatment methods.
Additional Challenge - Antibiotic Development :
One of the reasons for this ongoing problem is the struggle to find new antibiotics, as developing them is both costly and time-consuming. At the onset of antibiotic development, thousands of drug candidates are tested. However, many of these drugs fail during each stage of testing, from test tube experiments to animal studies and clinical trials, with only a handful making it to patients. Reducing the trial-and-error process is critical to saving time and money while improving treatment options.
RESEARCH
Antibiotic resistance poses a substantial global health threat . Leading academics have recently declared that we stand at the precipice of the “post-antibiotic era” . While limiting inappropriate prescribing of existing drugs and accelerating the development of novel antibiotics are key elements of any strategy to circumvent resistance, there is also a clear need to develop better treatment strategies using existing drugs to improve their efficacy and prevent the selection of further resistance.
Although antibiotics have been used for more than 70 years, we are not yet able to predict how antibiotic concentration affects activity (i.e. antibiotic pharmacodynamics) even in the simplest settings, e.g. E. coli growth in vitro. Our inability to design rational treatment strategies is illustrated by the substantial improvements in treatment that have been made solely based on expert opinion even after decades of clinical practice. Currently, most dosing recommendations are based on those regimens that perform best during a lengthy and expensive series of trial-and-error experiments. Many drug candidates fail during this testing process, and for those candidates that do make it through, the best regimen may well be missed. This trial-and-error approach also limits opportunities for the improvement of dosing for existing drugs and may slow down the development of new promising antibiotics. Rational dosing of new combination regimens using multiple drugs is even more complex. Antibiotic synergy and antagonism cannot usually be predicted and the nature of the drug-drug interaction may change depending on drug concentration. Furthermore, differences in the pharmacokinetic and pharmacodynamic profiles of drugs used in combination can facilitate the selection of resistance during multi-drug treatment.
There are many reasons that the development of new drugs is slow and expensive. While many of these are unavoidable and associated with ensuring safety and efficacy, delays and additional costs are partially attributable to bottlenecks that occur as candidate compounds are weeded out during library screening, pre-clinical research, and clinical trials. Late failures of drug candidates are especially problematic given the time and financial investments made during the long development process. These late failures are often due to relapse, either because of persisting bacteria or resistance evolution, an outcome that early trials of drug efficacy are not designed to assess. For example, in the recent trial assessing moxifloxacin for TB, despite excellent early success, patients were more likely to relapse with the new regimen compared to standard therapy.
We require new tools to rationally design dosing regimens to maximize the benefits of existing antibiotics and to shorten the development process for new antibiotics . The development of models that can inform optimal dosing strategies from data collected in early phases of antibiotic development (e.g. drug-target binding and transmembrane permeability) could accelerate the drug development process and help to identify promising compounds that should be prioritized. In particular, mathematical models that predict relapse from pre-clinical and early clinical data would be tremendously helpful.
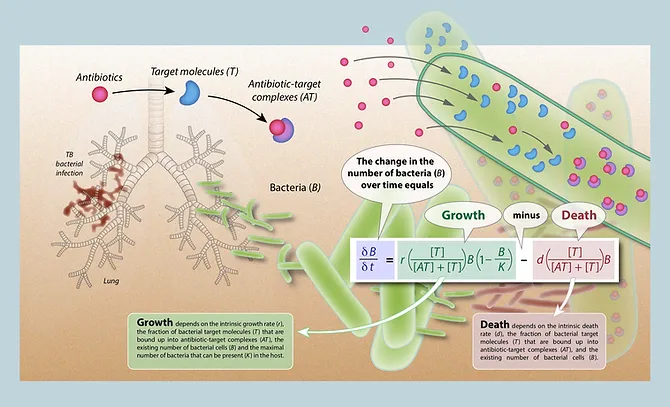
We use a novel mechanistic modeling framework that makes explicit links between chemical reaction kinetics (i.e. drug-target association and dissociation), effects on bacterial growth and death, and the population dynamics of bacteria within infected hosts. This modeling framework has been able to reproduce (and mechanistically explain) observed differences in antibiotic pharmacodynamics. Our mechanistic models differs from previous models, which have explicitly built in these pharmacodynamic effects.
Research interests
Antibiotics
Population Biology
Tuberculosis
Mathematical Modeling
Biochemistry
Pharmacology
Infectious Diseases
Where To find Us :